Equipment / facilities
Equipment / facilities
The group is well equipped to carry out synthesis, photophysical measurements and molecular modelling/calculations. Combining this range of facilities within a group allows us to design, synthesise and then study the properties of new materials. We are very fortunate in Durham to have access to a wide range of infrastructure/support which gives us a significant advantage over ‘single-method’ groups. We are particularly proud of our ability to investigate the optical spectroscopy of materials, and can claim to have studied just about everything – from budgerigar feathers to quantum dots! We routinely study samples in both solution and solid states, including thin films and can operate in inert atmospheres and record data over a wide temperature range (77-370K routinely. In addition to conventional steady-state UV-vis and fluorescence spectrometers we also have the following spectrometers:
New Toys!!
Recently we have added three major new items of instrumentation to the lab.
Luminescence Microscope
This system is based around an Olympus inverted microscope. It is equipped with CW and pulse excitation sources, both high sensitivity (long exposure) and video CCD cameras, a time-gated image intensifier for time-resolved imaging of long-lived fluors and a fibre coupled spectrometer capable of recording emission spectra from small sample areas.

Raman Microscope
We have just taken delivery of a Jobin-Yvon LabRamHR. This confocal scanning microscope is capable of recording routine Raman spectra and hyperspectral images of samples. It is equipped with a number of laser excitation sources.

Titanium-Sapphire Laser
A new addition to the laboratory the Coherent Verdi-pumped MIRA-D is equipped with a cavity dumper and 2nd/3rd harmonic generator. It provides tunable ps and fs pulses at variable repletion rates. This laser is destined to act as a UV and multi-photon excitation source for the time-correlated single photon counting spectrometer (in background). We have recently added the ability to routinely determine 2-photon cross-sections to this apparatus.

The group also has a Jobin-Yvon Fluorolog FL-3-22/ Tau-3 Spectrofluorimeter. This can record spectra over a wide range (250-1800nm) and can also record lifetimes (1ms-50ps). It operates on the phase-modulation principle and complements our time-correlated single photon counting spectrometer nicely. Like all of our instrumentation it is equipped to take a range of accessories including a fibre optic probe, integrating sphere and cryostat, allowing us to study a wide range of samples under various conditions. The addition of the integrating sphere is particularly useful for the absolute determination of fluorescence quantum yield of both solid and solution state samples, (PLQY), (J. Fluor. 2006 16 267-73).


The Oxford Instruments DN1704 cryostat installed in the Fluorolog, used for the study of samples down to 77K.
Combined Near-IR spectrofluorimeter / Laser flash photolysis
This versatile system is based around a Jobin-Yvon-Triax 320 which is located underneath the table supporting the scope etc. By simply repositioning a set of lenses it can be converted from a transient absorption to emission spectometer. The monochromator is equipped with gratings optimised for both UV-vis and NIR operation. It has two detector ports installed: one has a permanently mounted PMT whilst the can accommodate a wide range of detectors including a liquid nitrogen cooled germanium detector (the large blue object in the picture) or Si-PIN, InGaAs and avalanche diodes. Together these offer high sensitivity across the range 300-1800nm and can also cover a wide temporal range.
Samples can be excited using either a CW xenon lamp/monochromator or by a range of CW laser sources. Q-switched Nd:YAG lasers (266, 355, 532 & 1064nm) or a Nd:YAG pumped dye laser (400-800nm) are used for time-resolved measurements samples.
The front view… looking towards the pump lasers
The rear-view, showing the sample chamber. At the time of taking the picture the sample was placed in a cell equipped with a Young’s tap, this can be replaced by the cryostats (300-77K).

What it can do…
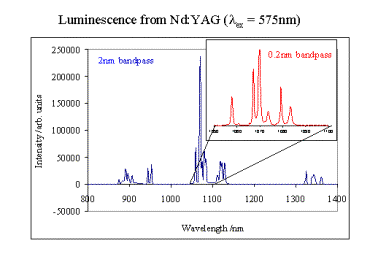
Steady state emission spectra
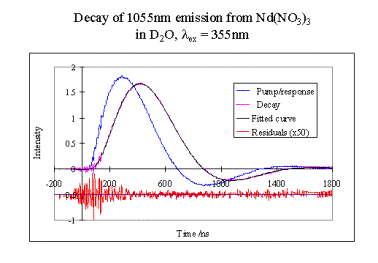
Time-resolved measurements – in this case the lifetime is 150ns. The excessive ‘ringing’ in the residuals between 0-200ns arises from the detector picking up some stray signal from the Q-switch of the laser.
Time-correlated single photon counting.

We also have a time-correlated single photon counting spectrometer. This equipment can routinely determine fluorescence lifetimes down to ca. 100ps. We currently have a range of IBH NanoLED sources including 370nm, 560nm (pulsed LEDs), 371, 396nm & 635nm (pulsed laser diodes), with the new Ti-sapphire laser waiting the wings to act as a deep UV source. The spectrometer is fitted with a cooled red-sensitive PMT (IBH TBX-04) which is good to 850+ nm and a single photon avalanche diode which is sensitive to ca. 1100nm. With the PMT the system has an instrument response function, after passage through the monochromator, of 1ns FWHM using the LEDs, 400ps using 635nm laser and 225ps with the 371 and 396nm laser diodes. The SPAD gives a slightly longer IRF but is still OK for lifetimes down to 100ps. The spectrometer can also be used to record time-resolved emission spectra, TRES.
A wide range of sample conditions can be accommodated: so far we’ve looked at solutions state samples (easy), temperature controlled solutions (325-77K), thin films (spin-coated & evaporated) and skin coated with sunscreen (challenging!).
The Jobin-Yvon Triax-190 monochromator is also equipped with a peltier cooled, 2096 pixel, linear CCD camera (built in-house, further details on request) for recording total emission spectra. This is controlled by a National Instruments NI-DAQ card and LabView software.
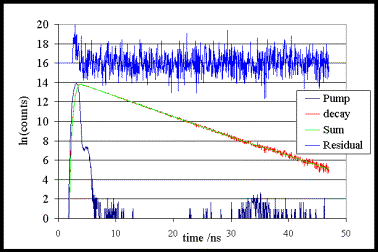
Fluorescence decay from ethanolic solution of menthyl anthranilate – a UV-A sunscreen. tf = 7.1ns, c2 = 1.05, excitation 370nm, emission 420nm. Note that the weighted residuals are shown offset on a linear scale.
We gratefully acknowledge the University of Durham, EPSRC and the Royal Society for funding.
Last modified 22/07/04 by A. Beeby
