Absorption Spectroscopy

3. Absorption Spectroscopy
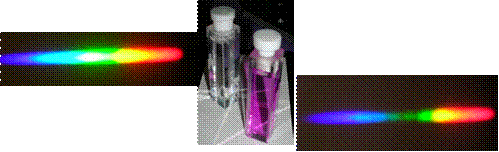
Through observing simple absorption spectroscopy, pupils can develop an appreciation for one of the underlying principles of spectroscopy, that coloured objects absorb certain wavelengths of the visible spectrum and transmit or reflect the others. In this example the spectrum on the upper left shows that of white light transmitted through a sample of pure water, and on the lower right a solution of aqueous potassium permanganate. The latter shows characteristic absorption of the green light.

The absorption of a sample is defined by the absorbance of a sample at a particular wavelength. The absorbance, A, is defined as: A = log10 (I0/It), where I0 & It refer to the intensity of light incident on the sample and transmitted by the sample respectively. The absorbance of a sample is related to the concentration of the absorbing species and the pathlength of the sample by the Beer –Lambert Law; A = e c l, where e is the molar extinction coefficient (mol-1 dm3 cm-1), c is the concentration (mol dm-3) and l is the pathlength (cm).
Using the RedTide spectrograph we can determine the absorption spectrum of a sample, plotting the absorbance of the sample as a function of wavelength. The RedTide is a combined spectrograph-CCD camera, and reads out the intensity of light entering via the fibre optic as a function of wavelength. To record an absorbance spectrum we need to record the spectrum of a blank sample, that is a cell containing pure solvent, and a spectrum of the sample itself. Calculation of absorbance as a function of wavelength is then carried out by the PC and spectrum plotted on the screen. A more detailed explanation of the procedure provided in the Spectroscopy in a Suitcase PowerPoint presentation.
A. Beeby, 04/09/08
